1. Yang Luo and Mark A Muesing. Prospective strategies for targeting HIV-1 integrase function. Future Med Chem. 2010 July 1; 2(7): 1055–1060. doi:10.4155/fmc.10.205.
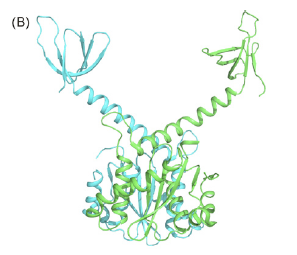
This review provides a good summary of the function and mechanism of HIV-1 Integrase. The protein has two catalytic activities: 3’ endonucleitic processing and strand transfer. The 3’ processing can occur in integrase’s dimer form, and the protein removes two nucleotides from the HIV viral DNA (vDNA) in order to prepare the vDNA for integration into the host genome. The next activity that occurs is the process of integration, and this requires a tetramer of the integrase protein (one dimer necessary to process each 3’ end of the vDNA). The end result of the catalysis is the insertion of the vDNA into the host genome, which allows for HIV to be replicated using host transcription machinery.
Integrase dimer
2. McColl, DJ, and Chen X. Strand transfer inhibitors of HIV-1 integrase: bringing IN a new era of antiretroviral therapy. Antiviral Res. 2010 Jan;85(1):101-18. Epub 2009 Nov 17.
This review provides a thorough analysis of the different inhibitors of the strand transfer reaction of HIV-1 integrase. The most promising of these inhibitors in the clinical setting are raltegravir and elvitegravir. These inhibitors act through the blockage of the active site of the integrase tetramer, or the catalytic core domain (CCD). This CCD is highly conserved within HIV-1, and provides an extremely effective target for antiretroviral therapy. The development of integrase inhibitors such as raltegravir has been extremely important to combat drug resistance in HIV-1, as the high mutation rate of the virus has lead to resistance to both protease inhibitors and reverse transcriptase inhibitors.
Raltegravir bound to the Catalytic Core Domain of Integrase
3. Sandy Azzi, Vincent Parissi, Richard G. Maroun, Pierre Eid, Olivier Mauffret, and Serge Fermandjian. The HIV-1 Integrase α4-Helix Involved in LTR-DNA Recognition Is also a Highly Antigenic Peptide Element. PLoS One. 2010; 5(12): e16001.
In this study, the activity of the α4-Helix of the integrase Catalytic Core Domain was found through a method that utilizes monoclonal antibodies. Taking the peptide fragment responsible for the α4-Helix, the researchers created monoclonal antibodies reactive to the peptide fragment by mouse immunization. These antibodies were first shown to be reactive to the α4-Helix found in the CCD. Once this was established, through competitive inhibition experiments the researcher were able to show that the CCD of integrase interacts with Long Terminal Repeats of the host DNA. Through such a technique, the researchers are able to further characterize the nature and location of the integrase CCD interaction with host DNA.



No comments:
Post a Comment