The first antiretroviral drugs developed were meant to target HIV protease and HIV reverse transcriptase activity. While moderately successful, it turns out that the high mutation rate of HIV led to the development of resistance to these drugs individually as quickly as 2 months post-treatment. In order to prevent this resistance combinatorial drug regimens of 3-5 different drugs had to be used, a regimen that is highly toxic to the body.
What does all this have to do with integrase you may ask? Well, it just so happens that integrase is another protein (along with protease and reverse transcriptase) that is necessary for viral replication. It also just so happens that it has become the greatest weakness to HIV's fitness.
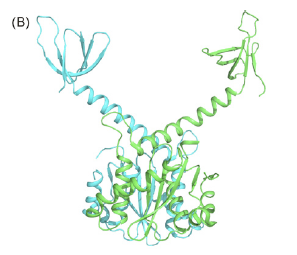
Here is a picture of integrase doing its thing: incoporating HIV DNA into the host genome (this is actually a tetramer of the enzyme):

Now, the regions of the enzyme that are bound to the DNA (yellow) are called catalytic core domains (CCDs), and they are necessary for the fuction of the integrase (see previous post). It also just so happens that these CCDs are highly conserved regions, meaning that they cannot be mutated or altered significantly without creating a disfunctional enzyme.
So, imagine the excitement of the HIV researching community when somebody did this:
 Do you see it? The space filling spheres and the protein cartoon are a zoomed in view of the CCD of integrase. The orange and blue stick diagram is a chemical called raltegravir. Raltegravir has been put on the market as an antiretroviral drug, because as this image is attempting to show, it binds quite will over the CCD of integrase, thereby inhibiting the DNA integration reaction.
Do you see it? The space filling spheres and the protein cartoon are a zoomed in view of the CCD of integrase. The orange and blue stick diagram is a chemical called raltegravir. Raltegravir has been put on the market as an antiretroviral drug, because as this image is attempting to show, it binds quite will over the CCD of integrase, thereby inhibiting the DNA integration reaction.
What's even more exciting, because the CCD of integrase has to be highly conserved to maintain function, far less resistance related to integrase inhibitors has been noticed in HIV+ patients, especially when used in a combinatorial drug regimen.
While integrase inhibitors are not effective enough to be used as a single drug for HAART, their effectiveness has allowed patients to be moved from HAART regimens of 4 or 5 drugs to only 2, a move that has reduced side effects considerably.
So here's to integrase: Because of its intricacy, because of the specificity of its CCD, we now have the most effective target for the treatment of HIV infection in the history of HIV research.
References:
1. Alian, A., Griner, S.L., Chiang, V., Tsiang, M., Jones, G., Birkus, G., Geleziunas, R., Leavitt, A.D., Stroud, R.M., 2009. Catalytically-active complex of HIV-1 integrase with a viral DNA substrate binds anti-integrase drugs. Proceedings of the National Academy of Sciences United States of America 106, 8192–8197.
2. Arribas, J.R., Pozniak, A.L., Gallant, J.E., Dejesus, E., Gazzard, B., Campo, R.E., Chen, S.S., McColl, D., Holmes, C.B., Enejosa, J., Toole, J.J., Cheng, A.K., 2008. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. Journal of Acquired Immune Deficiency Syndromes (JAIDS) 47, 74–78.
3. Bartlett, J.A., Chen, S.S., Quinn, J.B., 2007. Comparative efficacy of nucleoside/ nucleotide reverse transcriptase inhibitors in combination with efavirenz: results of a systematic overview. HIV Clinical Trials 8, 221–226.
4. Sherman, P.A., Fyfe, J.A., 1990. Human immunodeficiency virus integration protein expressed in Escherichia coli possesses selective DNA cleaving activity. Proceedings of the National Academy of Sciences United States of America 87, 5119–5123.